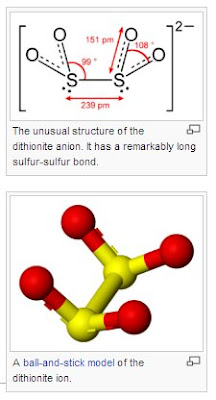
1. J. Organometal. Chem., 1975: Title: SYNTHESIS OF A TRANSITION METAL-DITHIONITE COMPLEX [pretty sketchy work but the first report] LINK
 2. J. Organometal. Chem., 1980: Title: SYNTHESIS, CHARACTERIZATION AND SOME REACTIONS OFORGANOMETALLIC COMPLEXES CONTAINING BRIDGNG DITHIONITE LIGAND [An extension of the previous publication to other metal systems] LINK
2. J. Organometal. Chem., 1980: Title: SYNTHESIS, CHARACTERIZATION AND SOME REACTIONS OFORGANOMETALLIC COMPLEXES CONTAINING BRIDGNG DITHIONITE LIGAND [An extension of the previous publication to other metal systems] LINK  3. Organometallics, 1985: Title: Reduction of SO2 by (C5R5)M(C0)3H (M = Mo, W; R = H, Me).Chemistry and Structures of (C5H5)Nlo(CO),(SO,H),the FirstExample of Insertion of SO2 into a M-H Bond, and[ (C5M5,)Mo(CO)3],(S2O4),S-Bonded Dithionite Complex [Complex is described by authors as "unexpected" but still recovered in 31% yield, note the complex is to the C2h dithionite structure and the S-S distance is 3.692 A] LINK
3. Organometallics, 1985: Title: Reduction of SO2 by (C5R5)M(C0)3H (M = Mo, W; R = H, Me).Chemistry and Structures of (C5H5)Nlo(CO),(SO,H),the FirstExample of Insertion of SO2 into a M-H Bond, and[ (C5M5,)Mo(CO)3],(S2O4),S-Bonded Dithionite Complex [Complex is described by authors as "unexpected" but still recovered in 31% yield, note the complex is to the C2h dithionite structure and the S-S distance is 3.692 A] LINK 4. J. Amer. Chem. Soc., 1999: Title: Oxidation of the Sulfide Ligands to SO42- in the Dinuclear Complex{RuCl[P(OMe)3]2}2(µ-S2)(µ-Cl)(µ-N2H4): Synthesis andCharacterization of {RuCl[P(OMe)3]2}2(µ-S2)(µ-Cl)(µ-N2H4)+HSO4-,{RuCl2[P(OMe)3]2}2(µ-S)(µ-N2H4), and{RuCl[P(OMe)3]2}2(µ-S2O5)(µ-N2H4) [The dithionite complex is more of an intermediate than an isolated product but the mechanism is amusing] LINK
4. J. Amer. Chem. Soc., 1999: Title: Oxidation of the Sulfide Ligands to SO42- in the Dinuclear Complex{RuCl[P(OMe)3]2}2(µ-S2)(µ-Cl)(µ-N2H4): Synthesis andCharacterization of {RuCl[P(OMe)3]2}2(µ-S2)(µ-Cl)(µ-N2H4)+HSO4-,{RuCl2[P(OMe)3]2}2(µ-S)(µ-N2H4), and{RuCl[P(OMe)3]2}2(µ-S2O5)(µ-N2H4) [The dithionite complex is more of an intermediate than an isolated product but the mechanism is amusing] LINK 5. Angew. Chemie, 2006: Title: Direct Observation of Photochromic Dynamics in the Crystalline State of an Organorhodium Dithionite Complex [Nice work but a classic case of duplicate publication, the S-S distance is 2.330 A and note the energetic similarity of the S-S and S-O bonded dithionites] LINK and LINK
5. Angew. Chemie, 2006: Title: Direct Observation of Photochromic Dynamics in the Crystalline State of an Organorhodium Dithionite Complex [Nice work but a classic case of duplicate publication, the S-S distance is 2.330 A and note the energetic similarity of the S-S and S-O bonded dithionites] LINK and LINK 6. J. Organometal. Chem., 2007: Title: Synthesis and structural characterization of a photoresponsive organodirhodium complex with active S–S bonds:[(CpPhRh)2(l-CH2)2(l-O2SSO2)] (CpPh = g5-C5Me4Ph) [it may seem like a simple extension but these complexes are pretty remarkable, S-S in cis is 2.295 A and S-S in trans is 2.299 A, how is that possible?] LINK
6. J. Organometal. Chem., 2007: Title: Synthesis and structural characterization of a photoresponsive organodirhodium complex with active S–S bonds:[(CpPhRh)2(l-CH2)2(l-O2SSO2)] (CpPh = g5-C5Me4Ph) [it may seem like a simple extension but these complexes are pretty remarkable, S-S in cis is 2.295 A and S-S in trans is 2.299 A, how is that possible?] LINK 

 4. J. Electrochem. Soc., 1995: Title: IN-SITU SPECTROELECTROCHEMICAL STUDIES ON THE SULFUR-DIOXIDE REDUCTION IN DIMETHYLSULFOXIDE []
4. J. Electrochem. Soc., 1995: Title: IN-SITU SPECTROELECTROCHEMICAL STUDIES ON THE SULFUR-DIOXIDE REDUCTION IN DIMETHYLSULFOXIDE [] 

 10. Chem. Phys. Lett., 2002: Title: Three-body dissociation dynamics of (SO2)3 studied
10. Chem. Phys. Lett., 2002: Title: Three-body dissociation dynamics of (SO2)3 studied




 6. Reactive Polymers, 1991: Title: REDUCTIONS WITH POLYMER SUPPORTED DITHIONITE ANIONS - REGIOSELECTIVITY IN CONJUGATED SYSTEMS [not exactly a soluble dithionite but ion exchange involving dithionite]
6. Reactive Polymers, 1991: Title: REDUCTIONS WITH POLYMER SUPPORTED DITHIONITE ANIONS - REGIOSELECTIVITY IN CONJUGATED SYSTEMS [not exactly a soluble dithionite but ion exchange involving dithionite]